MGFB
- Exploiting a novel therapeutic cancer vaccine platform
- Unique combination of xenogenic tumor derived cDNA library of antigens and APOBEC induced mutagenesis (HEAT: Heteroclitic Epitope Activated Therapy)
- Ph 1 study conducted in 12 uveal melanoma patients resistant to ICIs
About Us
Who We Are
MGFB was established in 2022 to develop therapeutic vaccines based on technology licensed from the Mayo Clinic. MGFB is a subsidiary of Fairwinds Bio.
This license from Mayo is based on 20+ years of research conducted by Dr. Richard Vile, a renowned scientist & world class leader in the field of adoptive cell and viro-immuno therapies.
The company intends to develop a pipeline of immuno-oncology therapeutic vaccines targeting multiple tumor indications.
Management Team

Ajit Gill
CEO, Founder, Director
Ajit Gill is past CEO and President of Nektar Therapeutics, when the company grew from a private startup to a public company with 800 employees and a market cap of over $2 billion USD. Mr. Gill graduated from IIT Kanpur with a B. Tech degree in Electrical Engineering and earned an M.S from University of Nebraska and an MBA from University of Western Ontario, Canada. Mr. Gill is currently serving as an advisor for several health care companies in India.

Sanjeev Munshi
PhD, MBA, CBO, Advisor
Sanjeev has over 25 years of experience in biomedical R&D, business development and alliance management, working in large biopharmaceutical and small biotech organizations. As an Executive Director of business development, Sanjeev led several in-licensing deals across multiple therapeutic areas, established external research collaborations, and managed alliances. Sanjeev holds a Ph.D. in Structural Biology from the Indian Institute of Science, India, and an MBA from Villanova University.

Chris Searcy
MBA, PharmD, CCDO
Chris has 35 years of industry experience working in a variety of settings from large multi-national pharmaceutical companies to early-stage therapeutic start-ups. Chris started his pharmaceutical career at Pfizer and spend 11 years in both technical and business roles. He earned his M.B.A. from the Wharton School at the University of Pennsylvania and has a Doctor of Pharmacy and Bachelor of Science from the University of Minnesota College of Pharmacy.

J. Andrew Sanford
MBA, CFO
Andy has over 30 years experience as a financial leader. Most recently he served as senior advisor to corporate executives and Board of Directors and founder of a financial consulting firm. Previously, served as Equity Capital Markets Group Head at Wells Fargo and was a member of the Investment Banking Operating Committee. Also worked as a Managing Director in Equity Capital Markets for J.P. Morgan Securities and Citigroup prior to that. Andy received a MBA from the Wharton School at the University of Pennsylvania and a BA from Dartmouth College.
Board of Directors

Ajit Gill
Founder and CEO
Ajit Gill is past CEO and President of Nektar Therapeutics, when the company grew from a private startup to a public company with 800 employees and a market cap of over $2 billion USD. Mr. Gill graduated from IIT Kanpur with a B. Tech degree in Electrical Engineering and earned an M.S from University of Nebraska and an MBA from University of Western Ontario, Canada. Mr. Gill is currently serving as an advisor for several health care companies in India.

Gloria R Olivier, Ph.D.
Director- High Value Opportunities | Business Development | Mayo Clinic

Sanjeev Munshi
PhD, MBA, CBO, Advisor
Sanjeev has over 25 years of experience in biomedical R&D, business development and alliance management, working in large biopharmaceutical and small biotech organizations. As an Executive Director of business development, Sanjeev led several in-licensing deals across multiple therapeutic areas, established external research collaborations, and managed alliances. Sanjeev holds a Ph.D. in Structural Biology from the Indian Institute of Science, India, and an MBA from Villanova University.
Scientific Advisors

Richard Geoffrey Vile, Ph.D.
- Published extensively (over 425 peer reviewed articles) in the field of immuno-therapy
- Dr. Vile’s research interests are focused on developing novel therapies for cancer, including adoptive T-cell and viro-immuno therapies. Over the past 20+ year’s Dr. Vile has developed novel oncolytic viruses with different payloads that can be delivered systemically to address tumor metastasis in combination with immune checkpoint inhibitors. Turning “cold tumors hot” and addressing “immune escape” by re-educating the immune system to recognize and react against tumor associated antigens is the key focus of his laboratory.
Technology
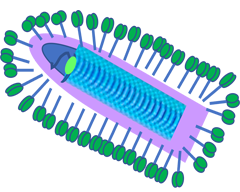
Addressing Tumor Resistance
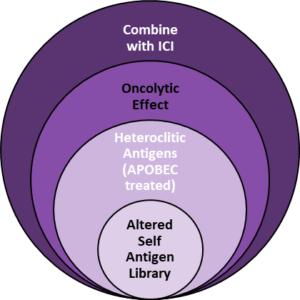
The platform is designed to address tumor resistance, immune escape and mutational burden commonly seen in many solid tumor patients. By delivering a broad repertoire of tumor associated antigens and neoantigens enhanced by APOBEC mutagenesis, strong T-cell responses have been demonstrated pre-clinically without the need for identification of patient specific tumor associated antigens. Preclinical studies in multiple tumor models including (Melanoma, GBM, HCC, Prostate). The results are consistent with strong and durable anti-tumor response when a library of Tumor Associated Antigents (TSSs) and APOBEC induced Tumor Specific Antigens (TSAs) are combined with Immune Checkpoint Inhibitors (ICIs).
Mayo has concluded a Ph1 trial in 12 uveal melanoma patients, results are pending.
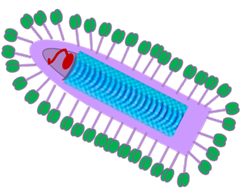
MFGB Recombinant VSV-IFNb based vaccine has the following features:
» Engages broad repertoire of tumor associated antigens (TAAs): a library of “altered self-antigens”, including APOBEC induced neoantigens
» Enhances abundance of weak TAAs, mounting a wide repertoire of weak anti-tumor T cell responses that collectively act to prevent tumor escape
» Increases the avidity of MHC class for antigen presentation
» Provides oncolytic activity and adjuvant effects
» Avoids need for identification of patient specific antigens or HLA type
» Activates cellular & adaptive immune subsets
» Compatible with IV, IT & Sub Q administration
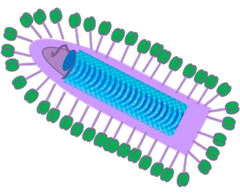
HEAT
Heteroclitic Epitope Activated Therapy Leverages APOBEC3B to Expand Antigen Repertoire
✓ Dysregulation of the APOBEC3B, a cytosine deaminase enzyme is a major contributor of DNA damage which drives the initiation and evolution of cancers either alone, or in the presence of response to different therapies
✓ HEAT utilizes APOBEC expression in tumor cells to drive heteroclitic epitopes
✓Library of heteroclitic antigens can prime the immune system to mount strong anti-tumor T-cell response
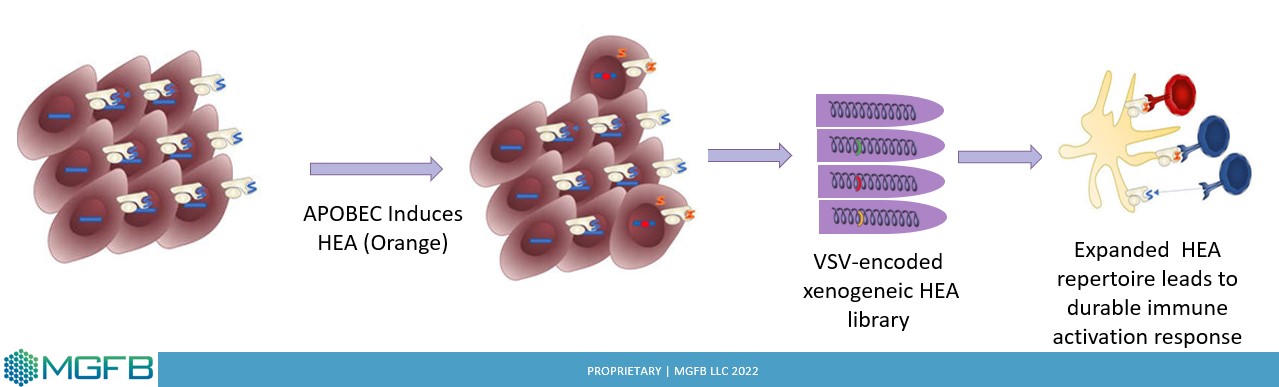
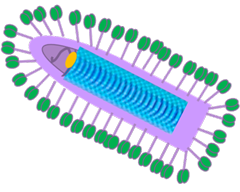
The Vaccine
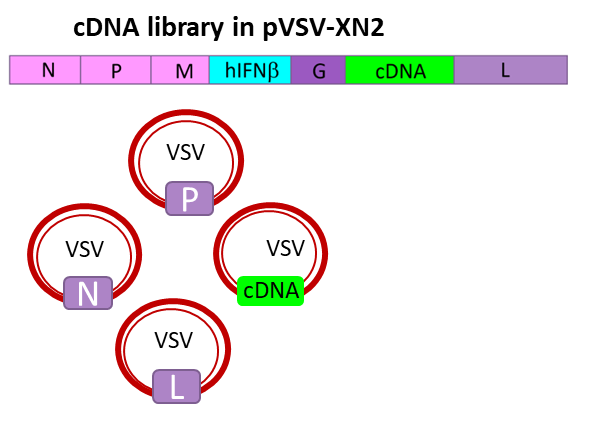
MGFB Vaccine
✓ MGFB Recombinant vaccine (VSV-IFNβ+cDNA library from tumor specific Xeno-explants that express APOBEC3B) addresses the challenges associated with treating solid tumors
Combining Multiple Vaccine Approaches into a Single Product to Deliver a Durable Response
Robust Anti-tumor response
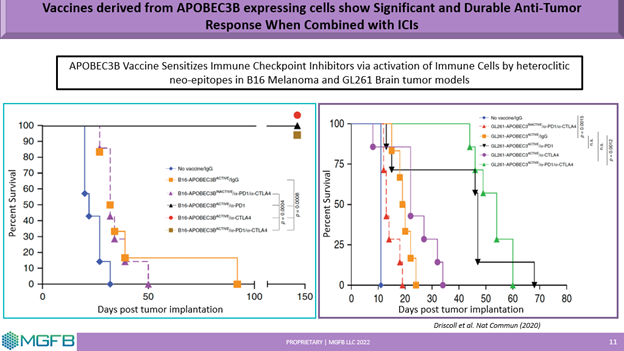
Select Publications
Media
Scientific
Pipeline
| Product | Tumor Target |
|---|---|
| M-001 | Melanoma |
| M-002 | Glioma |
| M-003 | Colorectal Cancer |
| M-004 | Hepatocellular Carcinoma |